Water Hardness and pH
Water is classified as either soft or hard:
- Soft water contains relatively few minerals and lathers easily.
- Hard water is rich in minerals such as calcium and magnesium, which is the cause of “scale” in kettles.
Water hardness is usually expressed as the number of parts per million (ppm) of calcium carbonate present in the water
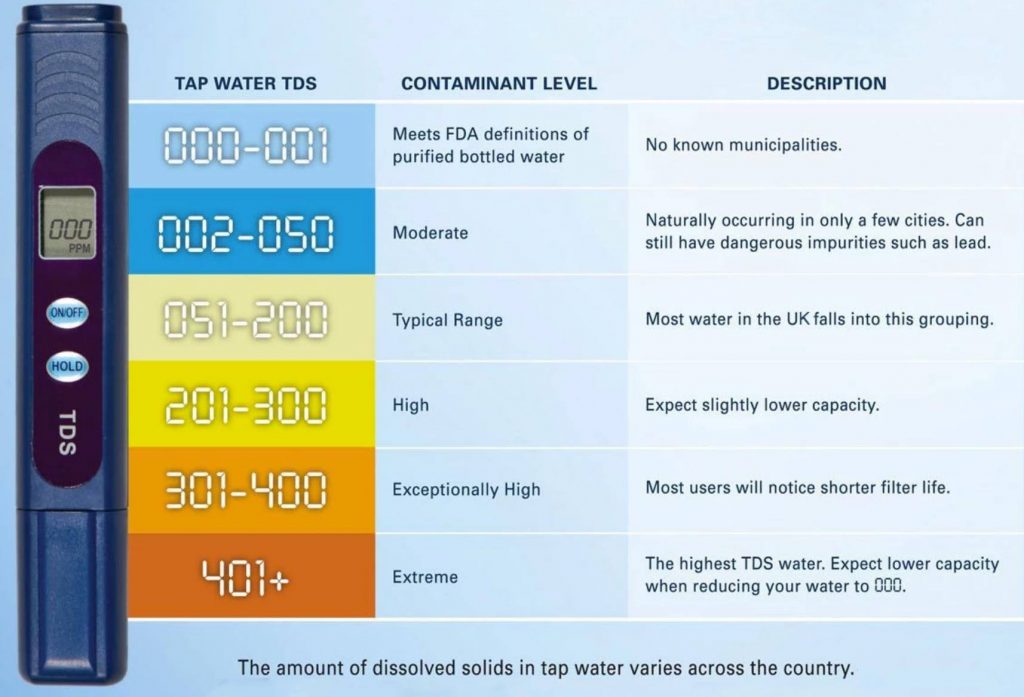
You can test your water hardness using TDS meter. Read more about TDS meters click here.
pH
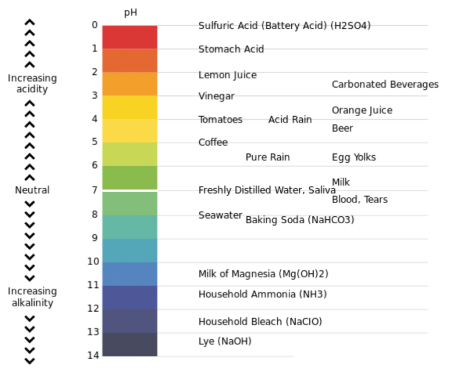
pH = hydrogen ion concentration. The scale ranges between 0 and 14
Importance of pH
The pH of water determines the solubility (amount that can be dissolved in the water) and biological availability (amount that can be utilized by aquatic life) of chemical constituents such as nutrients (phosphorus, nitrogen, and carbon) and heavy metals (lead, copper, cadmium, etc.). For example, in addition to affecting how much and what form of phosphorus is most abundant in the water, pH also determines whether aquatic life can use it. In the case of heavy metals, the degree to which they are soluble determines their toxicity. Metals tend to be more toxic at lower pH because they are more soluble
In general, a water with a pH < 7 is considered acidic and with a pH > 7 is considered basic. The normal range for pH in surface water systems is 6.5 to 8.5 and for groundwater systems 6 to 8.5. Alkalinity is a measure of the capacity of the water to resists a change in pH that would tend to make the water more acidic. The measurement of alkalinity and pH is needed to determine the corrosive of the water.
Test your water using – Digital Electric PH Conductivity Meter Tester

Or PH Tester strips

Although pH and hardness are different properties of water, they are closely linked. Whilst pH is a measure of the acidity and alkalinity of water, hardness is a measure of the dissolved minerals in the water.
